Treating Rust Using Electrolysis

By Andrew Westcott
-- Links to other pages I've written --
Home Page
Radio Stuff
Concert Master
Coxparo
Gin Traps
Grimspound
Rust Electrolysis
National Explosives
Solar Hot Water
Wistmans Wood
Doddi Mines
Rants
Dog Walker
Zombies!
JS Email
Telephone Intercom
Whetstone Mining
Pulsar Zero 2250
Index Of Sub-Sections On This Page:
Introduction To Electrolysis
Safety Considerations
Setting Up An Electrolysis Cell
Special Considerations
Examples Of Treated Artefacts
Further Reading
Introduction To Conserving Iron & Steel Artefacts
Rust electrolysis is the process of using electricity to treat rusted iron and steel components.
Iron and steel artefacts which have been buried in the ground for any significant length of time will invariably be in a badly corroded condition, but electricity in conjunction with an electrolyte can be used to partially restore such items as long as a substantial iron core remains and the treatment is appropriate for the artefact; this process is known as rust electrolysis.
In such badly corroded finds there will be, in the simplest terms, two types of rust present: the outer 'red rust' layer and a deeper 'black rust' formation. The outer 'red rust' component takes up more volume than the metal it originated from and tends to loosely adhere to the main body. This flaky layer represents a total loss of metal from the artefact and can't be recovered although electrolysis treatment does usually make it easier to remove.

Equipment for a small electrolysis cell
Beneath this outer layer of red rust there is generally a substantial layer of 'black rust' which formed in the oxygen-deprived region underneath the outer corrosion. Unlike the surface rust, this underlying corrosion product takes up the same volume as the original iron and is tightly bound to it; it's possible for some of this underlying 'black rust' to be converted to a stable material with careful treatment in an electrochemical cell, allowing the preservation of much of the artefact's original dimensions. The chemistry behind iron oxidation is complex, but this site may help explain it: Chemistry Of Rust.
The idea of using electricity to treat rusted iron isn't new, and electrolysis has been used for the conservation of ferrous artefacts by collectors and archæologists for many decades. The results can be impressive with the substantial preservation of the original surface, but the exact requirements are sometimes poorly understood and the suggested equipment often crude, exacerbated by the many web pages out there offering poor or misleading advice on this delicate process.
Why use electrolysis when there are simpler methods?
Iron & steel items can be sandblasted or treated with acids to remove the rust and this certainly works, but the valuable underlying 'black rust' which may still retain surface details is also removed, resulting in that part of the artefact being lost for good. Electrolysis offers the possibility of recovering the iron which has turned to black rust, which is why the process is so important for the conservation of valuable and rare artefacts.
Rust electrolysis is not some magical, or quick and easy way of cleaning rusty items and is generally only to be considered for post-mediaeval artefacts with a substantial iron core remaining, as more severely corroded finds may need a different preservation approach. The amount and type of corrosion, coupled with varying degrees of adhering encrustation can all contribute to a variable outcome and needs to be considered.
Back to top
Safety Considerations

The electrolyte used is mildly alkaline (washing soda) and although not considered dangerous, prolonged contact with the skin is best avoided as it is an irritant. Obviously, if it gets into your eyes, wash it out with plenty of water and get medical advice.
The equipment needed for electrolytic rust removal involves the use of electricity, although the voltages usually encountered in electrolysis are not considered dangerous. However, if the hands have been immersed in electrolyte for some time and the skin has become highly conductive, even a relatively low voltage may allow significant current to flow through the body, so it's wise to switch off the supply before putting your hands in the solution, should you need to. There is obviously also a concern with mains equipment being sited close to conductive liquid, so use a bit of common sense regarding the relative positioning of the components.
The production of explosive gasses is something to be aware of: even when using sensibly low currents, some hydrogen and oxygen will be evolved at the electrodes, producing a potentially explosive mixture. The procedure is best conducted in a well ventilated area and sparks or flames in the vicinity should be avoided. Sparking of the electrode connectors can, interestingly enough, be eliminated by switching off the power supply first.
Back to top
Setting Up An Electrolysis Cell
The following sections deal with setting up an electrolysis cell in detail, and should act as a good guide to effectively treating a moderately rusted artefact.
Pre-treatment Artefact Care
It is important that a rusted artefact is not allowed to dry out once removed from the ground, so it should be immediately sealed in an airtight bag upon removal to keep it damp until it can be dealt with, which should be at the earliest opportunity.
Now a decision needs to be made as to what treatment the artefact will undergo:
ELECTROLYTIC TREATMENT
If electrolysis is planned, this should begin as soon as possible; note that not all iron artefacts are suitable for this treatment.

Testing the pH
NON-ELECTROLYTIC CLEANING
If a mechanical form of cleaning is intended and there is going to be a delay before this can be done, it is considered that "iron is best protected in an aqueous solution with 9-13.5 pH range." (The Electrochemical Society). Although this probably won't stop rust advancing completely, it does slow it down considerably.
For reference, here are some real-world tested pH values for various strengths of Na₂CO₃ . 10H₂O
(Sodium carbonate decahydrate):
1% w/v = 11.29
2% w/v = 11.40
4% w/v = 11.45
IMPORTANT!
Do not store artefacts in an alkaline solution if electrolysis treatment is planned; this seems to cause chemical changes to the 'black rust' which then no longer seems to be reduced by electrolysis, rendering it largely ineffective. I'm unsure why this is, and I'm conducting further tests to verify the observation and to find out why.
The Container

A suitable container
A suitable container for the electrolyte is needed which is large enough to totally immerse the artefact, and the obvious choice would be one made of plastic, as this is entirely inert and non-conductive. For later on, you will need to know how much liquid is required to fill the container to the required level, and this can be done by filling it with water using a graduated jug, noting the total volume needed.
The Power Supply

Old, but perfect for the job
You are going to need a source of power for this process, so get your hands on a suitable regulated power supply with current limiting; as this component is central to the operation, get something suitable, they aren't that expensive if you are serious. The voltage should be above around 5 volts but not so high as to be a hazard, so one offering 30 volts at 1 amp or thereabouts would be a good choice, and such units are widely available on the second-hand market.
The equipment should be able to allow the current to be smoothly limited down to a few milliamps, this being the most important requirement. A maximum rating of 1 amp is more than enough for this process unless you are expecting to treat something particularly large.
If you intend to use a power supply that doesn't have current limiting or a variable voltage, you could use a selection of resistances to place in series with the supply to do the current limiting: a resistance of 220Ω would limit current to around 50mA with a 12 volt supply, for example, but having fixed resistances does limit the adjustability of the equipment.
If your artefact has any value, either historical or personal, do not connect a battery charger directly to the electrodes; I see this suggested on many web sites but it is very poor practice and demonstrates a fundamental lack of understanding of the electrolytic process. An unmodified battery charger will not do for conservation work, don't use one.
To explain, there are two major issues with using a battery charger: firstly the low resistance of the electrolyte could allow large currents to flow causing a real risk of damage to the charger, or even fire. Secondly, the resulting current flow is far too high for effective iron recovery as the high rate of gas formation will damage the surface of the artefact, loosening corrosion products which may have otherwise have been recoverable. In addition to this, the large quantities of explosive gasses produced are a real danger, the anodes will corrode badly and the electrolyte will become a mess.
Anode Construction
Suitable material for the anodes needs to be obtained, and 0.6mm or 1mm plain steel sheet as used for car repairs is an excellent choice as it can easily be cut and shaped and is inexpensive, but ensure it isn't galvanised.
Metal sheet like this is often coated in a protective film of oil and this layer needs to be removed with a solvent before use, and obviously it must not be painted: anything which may inhibit the flow of electricity between the metal plates and the electrolyte has to be removed. I have found that even the thinnest layer of oil or grease (which you may not even be aware of) will insulate that part of the plate, so I recommend giving the surfaces a good sanding to ensure bare metal.

Anode plates fitted within the container
The anode plates should be cut and shaped to fit around the interior of the container, including one across the bottom, ensuring part of each plate protrudes above the water level to enable a connection to be made. All of the anode sections must of course be electrically linked to each other using clips and wire.
There are web pages out there suggesting using a bit of steel rod for the anode, but if you are serious about conserving your artefact, do it properly and prepare some decent anodes to surround it; the process will run far more predictably and reliably.
Suspending And Connecting The Artefact
Once the container and anode plates have been prepared, attention must turn to how you can securely suspend the artefact, and how to make an electrical connection to it: it should go without saying that this is best done before adding the electrolyte.

A dry run: suspending the artefact
The best material to use for suspending the artefact is thin string, as it is porous and won't affect the process where it is in contact with the artefact; the string can be tied to thin strips of wood resting across the anodes or the container. Experiment to get the suspension height within the container correct: it shouldn't be too close to the bottom plate and obviously should be below the intended surface of the electrolyte.
The artefact needs to be prepared for treatment by using a file or similar to carefully remove just enough rust on one edge in order to expose a small area of metal, the bare minimum required for an electrical connection to be made using a crocodile clip. This is a destructive action but critical to the process with no alternative, so the site of the proposed metal exposure must be chosen carefully.
A solid connection to the artefact is vital to the process, so this connection should be verified as sound; the only way to confirm continuity with total certainty is to expose the smallest area of bare metal on a different part of the artefact and check that current can be passed between the two points, through the metal of the artefact.
Note that if the artefact consists of more than one component, for example a gin trap, all of the separate components need to be electrically connected to the cathode. This is important; don't assume the components will be electrically linked just because they are touching each other.
Suspend the artefact in the chosen position, make the electrical connection to it and verify that connection as just described; once the connection has been confirmed, be careful not to move the artefact or the connection to it at all from this point on as this could disturb that all-important electrical continuity.
Introducing The Electrolyte
Hopefully, you measured the volume of the container earlier so know how many litres of electrolyte are required.

An alkaline electrolyte can now be prepared, and I consider the best all-round option to be a solution of sodium carbonate (Na₂CO₃) as it is reasonably safe and also readily available at many supermarkets as washing soda or soda crystals. Some professional conservation laboratories suggest using a 2 - 5% solution of caustic soda (NaOH), and while this may be beneficial in helping conserve artefacts recovered from a marine environment, it is a dangerous chemical, needs careful handling and as such may not be appropriate for the home experimenter.
I use a 2% w/v solution of sodium carbonate which seems to work well, producing an alkaline environment and being highly conductive while not using excessive amounts of chemical. In order to demystify quoted solution strengths, it simply indicates the weight of chemical contained in 100mL of solution, so for example a 2% solution would involve dissolving 2 grams of chemical in water and making up the final volume to 100mL. This is obviously the same as 1 litre containing 20 grams of chemical, 20 litres containing 400 grams and so on.
It's sensible to first dissolve the required amount of chemical in a few litres of water in a bucket or similar rather than trying to dissolve it in the electrolysis container, as the stirring required may result in the connection to the artefact being disturbed. Once prepared, the solution can be carefully poured into the electrolysis cell, being very careful not to disturb that vital electrical connection to the artefact; continue slowly until all of the dissolved chemical is in the container, finally topping up with water to the required level.
I've found it can be tedious trying to end up with a clear solution, so don't worry about this minor detail; I've noticed that despite using rain water, a small amount of white insoluble material settles out, which is presumably due to impurities present in the sodium carbonate. In hard water areas, a precipitation of white calcium carbonate may be noticed and although unsightly, it is of no consequence; it doesn't affect the outcome at all and will settle to the bottom of the container eventually.
Applying The Power
This is the most critical phase and, it seems, the least understood.
Ensure all of the anode sections are linked together, and connect them to the positive output of your power supply. The connection to the artefact must go to the negative connection.

An artefact undergoing treatment
The current should be set to a value appropriate for the size of the artefact, meaning ones with a larger surface area can accept a higher current and still be safely treated. A rough guide I've seen suggested is to use around 1mA of current for each square centimetre of surface area, although I would suggest perhaps a quarter of that.
A more accurate method is to measure the voltage developed across the electrodes using a digital multimeter, which gives an excellent indication of the suitability of the current.
By way of example, the heavily encrusted artefact pictured above has an area of around 140cm², and for a 2·1 volt reading needed a current of around 42mA, amounting to about 0·3mA/cm². The current required to develop this voltage fell slightly as treatment progressed.
Here is a simplified and standardised treatment regime I've found to be effective in most cases, based on the voltage developed across the electrodes.
• Initially, set current to give 1 volt, then keep that current fixed. Run for a day or so.
This is the stabilisation phase.
The voltage will rise initially but it will become more stable over time; if it reaches 2·1 volts, reduce current to keep to that maximum voltage.
• Set current to give 2·1 volts; run for 4 days or so.
This is the main conversion phase and can take several days to complete.
Maintain this voltage by reducing the current as needed; there should be no obvious gas production during the early part of this phase, and parts of the corrosion may darken in colour.
• Set current to give 2·2 volts; run for 2 days or so.
This is the finishing phase which ensures the process is complete.
Maintain this voltage by adjusting the current as needed; there may some slight gassing visible at this stage.
Note that if the voltage unexpectedly increases by a volt or more for the same current and you see gassing from the crocodile clip, suspect the connection to the artefact and correct it immediately.
Be patient, and allow the process to run its course. Regularly monitor the voltage to be sure you are maintaining a good connection and that the voltage across the electrodes isn't climbing too high, causing excessive gassing which could be detrimental to the process.
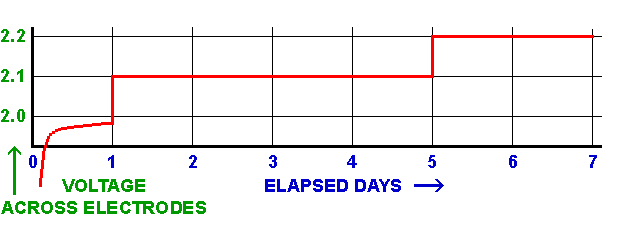
Graphical representation of my electrolysis treatment regime
The voltages suggested above produce the necessary potential and H⁺ ions for cathodic reduction, without causing unnecessary evolution of hydrogen gas in the early stages, which would hinder the process and risk loosening recoverable rust deposits. When a few bubbles start being noticed at the cathode, this indicates that most of the rust reduction which is going to occur, has already happened but continue the treatment for a few days longer to be sure.
Final Cleaning
Once an appropriate length of time has elapsed, it will be time to switch off the power and remove the artefact from the electrolyte.
The artefact now needs to be washed to remove all traces of the electrolyte, and at the same time can be given a good scrub using a stiff plastic brush to remove loose deposits from the surface. Once most of the easily removable deposits have been removed let the artefact sit in cold water for a few hours, changing the water occasionally to be sure the electrolyte has been completely washed off and has had a chance to leach out of the pores in the metal.
Give the artefact a good final wash and then quickly dry it off. This is important, as the porous recovered iron has a tendency to rapidly rust, in a matter of minutes sometimes. It's worthwhile immersing the artefact in a water-miscible solvent such as alcohol or acetone for a while, to help draw the water from the pores in the metal to ensure none is left, then heating the artefact up using a hair dryer to quickly dry off the solvent before it absorbs moisture from the air. The amount of attention you give at this stage should relate to how much value you place on the artefact; some items simply don't warrant too much attention to detail.
Put the artefact somewhere warm for a few days to ensure it is completely dry, then you can get to work with a small wire brush to remove the remaining deposits, and depending on the exact composition of the original iron, you should be able to uncover the original surface of the artefact, now preserved in a graphite-coloured material, mainly Fe₃O₄ and some metallic iron. If there are stubborn crusts of rust still adhering, you can gently try to dislodge them using a sharp tool but be careful not to flake off that fragile surface.
Despite the precautions, you may find new areas of red rust developing; the best you can do is to remove what you can of it. Use a mildly volatile solvent such as paint thinner or petrol to wash the dust off the artefact; don't use water this time as you will risk ongoing corrosion because the water will enter the pores in the recovered iron. Once washed and the solvent fully evaporated, the artefact should be given a coat of preservative such as Hammerite's Waxoyl, one of Fuch's preservatives from the Anticorit range or just good old oil. Ongoing corrosion is a real problem with a treated artefact, so any anti-rust treatment needs to be checked regularly and the treatment re-applied as necessary to maintain protection.
Back to top
Special Considerations
Voltage And Current
This issue has been dealt with earlier, but it's so important that I'll mention it again: the supply voltage is not critical, as long as it is higher than around 5 volts and not so high as to be a health hazard. If too high a current is allowed to flow, any deposited iron will be very porous and possibly become detached from the surface and the rapid hydrogen bubble production can blast off rusted metal which could possibly have been recovered using much lower currents. If you are finding the anodes rust badly and the electrolyte bubbles a lot and becomes discoloured, the current is far too high and you may just as well have sandblasted the item.
Electrolysis Can Damage Paint
Be aware that electrolysis can soften and lift paint; this may be due to the original surface not being properly prepared before painting, or possibly because corrosion had crept under the paint layer. Certain paints may also be softened by the electrolyte itself. So be warned that if you have an object of value with a painted surface, and if that paint is important to its value, consider an alternative to this process.
Hydrogen Embrittlement
There is a known process by which steel can absorb hydrogen during the electrolysis process, which is known to cause springs to become brittle. This change is considered temporary and will eventually reverse if left alone, or the process can be speeded up by heating the item in an oven. The best advice is, don't operate any springs on recently cleaned items to eliminate the chance of breakages. More about it here: Hydrogen embrittlement
Painting The Treated Item
I have received a fair bit of communication regarding whether the surface of the treated metal is suitable for painting without further treatment. I use electrolysis to reveal surface features so I don't cover that with paint. However, I would suggest a coating of 'red lead' primer followed by a conventional metal paint should do the job and that there would be little chance of rust forming under the paint as long as the artefact was well rinsed and thoroughly dried beforehand.
Examples Of Treated Artefacts
The pairs of photos here show examples of artefacts I have treated using electrolysis, to illustrate what to expect and what can go wrong. As should be obvious, once iron has rusted away, it's gone for good and nothing will recover it, but this process does convert some rust to a stable material and makes it easier to remove oxidation which couldn't be safely removed otherwise.

Before |

After |
This horse shoe was in a particularly badly rusted condition, having spent much of its time buried in the ground. Unfortunately it had been allowed to dry out, causing the rust layers to separate from the core. It was treated for around a week in the cell, but if it hadn't been allowed to dry out before treatment, some of the corroded iron may have been recoverable; as it is, I was left with pretty much just the iron core in this case.

Before |

After |
The photos above show a badly corroded and damaged tool which was buried in the ground for around 200 years, and how it looks after careful treatment for a week or so in an electrolysis cell and some careful cleaning; I consider this to be a good result and as long as conserved artefacts like this are kept moistened with an anti-rust fluid, they should last indefinitely. Museums take note!
Back to top
Further Reading
Archaeological Metal Artefact Reduction/Cleaning By Electrolysis
Research into electrolytic conservation of iron artefacts.
Overview Of Archaeological Iron
A chemistry-heavy advanced paper written on the conservation of archaeological iron.
Freshly Excavated Iron Artifacts
Investigation into the Potential of Low-Oxygen and Dry/Cold Storage for Freshly Excavated Iron Artifacts.
Artefact Preservation Equipment
Useful stuff for archaeological finds.
I can be contacted at this address:
Copyright © Andrew Westcott 2003 - 2026
I'm happy for anyone to use this material for private, non-commercial or educational purposes, but credit to the author must be given. For any other use please contact me.
No cookies. No Tracking. No AI text. No ads.